Additional Information
The Additional Information section of the Protocol page allows for the entry, maintenance and viewing of non-required, general information about the protocol. Although optional, these are standard fields that most implementing institutions are likely to collect. They allow for specification of additional identifying information about the protocol and selection of applicable areas of research.
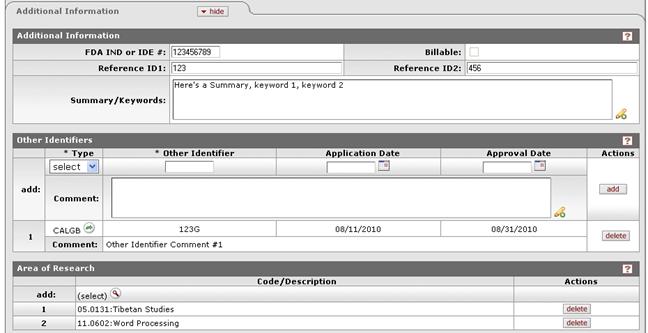
Figure 1106 Protocol Document, Protocol Page, Additional Information Section – All Subsections Example
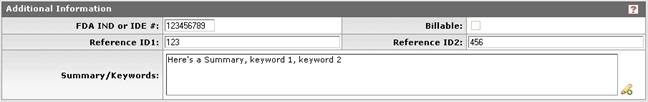
Figure 1107 Protocol Document, Protocol Page, Additional Information Subsection Example
Table 322 Protocol Document, Protocol Page, Additional Information Section Field Descriptions
|
Field |
Description |
|
FDA IND or IDE # |
Type to enter the U.S. Food and Drug Administration (FDA) Investigational New Drug (IND) application or Investigational Device Exemption (IDE) approval number when applicable. |
|
Reference ID1 |
Type to enter an alphanumeric (spaces & special characters allowed) identification reference to track the protocol as defined by your institution or sponsor (field label may differ from example shown based on your institution’s unique implementation).
|
|
Summary/Keywords |
To briefly describe the research, click within the
text box (or press the tab |
|
Reference ID2 |
Type to enter an alphanumeric (spaces & special characters allowed) identification reference to track the protocol as defined by your institution or sponsor (field label may differ from example shown based on your institution’s unique implementation). |

 key from a previous field) to
relocate the cursor to the field, and then type (or paste from virtual
clipboard) to enter text in the box as necessary to provide the
appropriate information.
key from a previous field) to
relocate the cursor to the field, and then type (or paste from virtual
clipboard) to enter text in the box as necessary to provide the
appropriate information. Other Identifiers
Other Identifiers